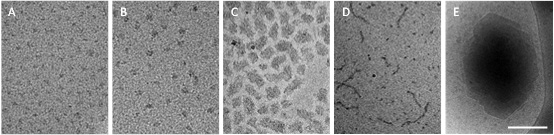
Nanocarriers based on self-assembled BETA-casein micelles
β-Casein is an amphiphilic protein that self-organizes above the critical micellar concentration (CMC) into well-defined core-shell micelles (Fig. 1A). In my research, I develop these protein micelles as efficient nanocapsules of drugs for oral delivery applications. Such core-shell polymeric micelles are considered very attractive candidates for drug delivery, as potentially they can encapsulate large loads of water-insoluble as well as amphiphilic drugs at the micelles’ hydrophobic core (Fig. 1B) or core–shell interface (Fig. 1 C and D), and provide enhanced stability, prolonged circulation times and controlled release.
At the same time, β-casein has all the essential molecular characteristics of an excellent emulsifying agent and polymeric stabilizer, thus it is also utilized for drug stabilization in the form of nanocrystals (Fig. 1E).
Fig. 1: β-Casein micelles: empty (A) and loaded with various poorly water-soluble drugs (B-E), showing its excellent and versatile abilities as a drug delivery platform. Celecoxib is solubilized, stabilized and protected in the micelle core at an amorphous state (B); Loading with ibuprofen leads to aggregation (C) or mixed micellization (D); Budesonide is stabilized as nanocrystals of well-defined size and shape. Bar = 100nm (A-B) and 200 nm (C-E)
Novel Cochleates for Nano-Medicine
Cochleates are lipid microstructures, consisting of negatively charged phospholipid bilayers, in which the planar phospholipid bilayer is induced to wrap around itself to form elongated multilayered cylindrical structures. A novel type of cochleate, able to microencapsulate water-soluble cationic drugs or peptides into its inter-lipid bilayer space, may be formed through interaction between negatively charged lipids and drugs or peptides acting as the inter-bilayer bridges instead of multi-cationic metal ions. The main goal of the research is to explore the conditions required to engineer efficient and stable nano-structures consisting of different species of therapeutic agents with a variety of lipid mixtures mimicking the cytoplasmic membrane of bacteria. Ultimately, to reveal the mechanisms dictating the drug-lipid conjugates assembly and develop advanced methodologies for controlling and characterizing the structures in terms of size, structure and morphology.