From Discs to Ribbons Networks
At the critical micelle concentration (CMC), amphiphiles self-assemble into spherical micelles, typically followed by a transition at the second CMC to cylindrical micelles that are uniform in width but are polydispersed in length and have swollen ends. We show a new structural path of self-assembly that is based on discoidal (coin-like), rather than spherical, geometry; the nonionic sterol ChEO10 is shown to form monodisperse equilibrium disc assemblies at the first CMC, transitioning at the second CMC into flat ribbons that (like the cylindrical micelles) have uniform width, polydispersed length, and swollen ends. Increase in ChEO10 concentration or the temperature leads to ribbon elongation, branching, and network formation. This self-assembly path reveals that (1) surfactants can form equilibrium nonspherical assemblies at the CMC and (2) aggregate progression around the second CMC is similar for the disc and sphere geometries.
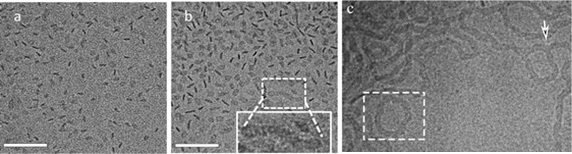
Figure: At 30 °C, 1 wt% ChEO10 disc-like aggregates are formed (a). (b) 2 wt %: coexistence between disc-like assemblies and some elongated, ribbon structures. The inset highlights a short ribbon with swollen endcaps. (c) At 50 °C, 1 wt%, network creation through three-fold junctions (white arrows) between ribbon segments, as well as rings (enclosed in rectangles). Scale bar = 100 nm
Internalization of Silica Nanoparticles into Fluid Liposomes: Formation of Interesting Hybrid Colloids
The formation of hybrid materials consisting of membrane-coated silica nanoparticles (SiNPs) concentrated within small unilamellar vesicles (SUVs) of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) is described. They are formed by a simple self-assembly process resulting from invagination of the SiNPs into the SUVs and subsequent vesicle fusion, thereby retaining an almost constant size. This process was followed under conditions where it proceeds slowly and could be analyzed in structural detail. The finally formed well-defined SiNP-filled vesicles are long-time stable hybrid colloids and their structure is conveniently controlled by the initial mixing ratio of SiNPs and vesicles.
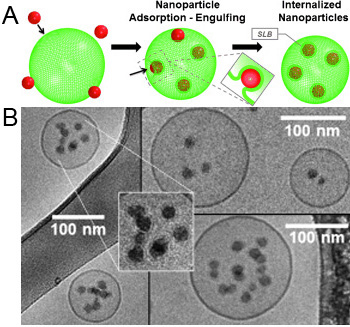
Figure: Mechanism of NP internalization. (A) Scheme depiction – hydrophilic NPs adsorb to the lipid are wrapped and finally internalized into the fluid liposomes, thereby forming supported lipid bilayer (SLB) NPs (the inset depicts the process of invagination). (B) Cryo-TEM micrographs showing NP internalized into vesicles. The enlargement shows the presence of SLBs around internalized SiNPs.
A simple route to fluids with photo-switchable viscosities based on a reversible transition between vesicles and wormlike micelles
Recently, there has been much interest in photorheological (PR) fluids, i.e., fluids whose rheological properties can be tuned by light. In particular, there is a need for simple, low-cost PR fluids that can be easily created using inexpensive, commercially available ingredients and that show substantial, reversible changes in rheology upon exposure to different wavelengths of light. Towards this end, we report a class of photoreversible PR fluids prepared by combining the azobenzene derivative 4-azobenzene carboxylic acid (ACA) (in its salt form) with the cationic surfactant erucyl bis(2-hydroxyethyl)methyl ammonium chloride (EHAC). We show that certain aqueous mixtures of EHAC and ACA, which are low-viscosity solutions at the outset, undergo nearly a million-fold increase in viscosity when irradiated with UV light. The same solutions revert to their initial viscosity when subsequently exposed to visible light. Using an array of techniques including UV-vis and NMR spectroscopies, small-angle neutron scattering (SANS) and cryo-transmission electron microscopy (cryo-TEM), we have comprehensively characterized these PR fluids at the molecular, nanostructural, and macroscopic scales. Initially, EHAC–ACA are self-assembled into unilamellar vesicles, which are discrete container structures and give the sample a low viscosity. Upon exposure to UV light, ACA undergoes a trans to cis photoisomerization, which alters the geometry of the EHAC–ACA complex. In turn, the molecules self-assemble into a different structure, viz. wormlike micelles, which are long, entangled chains and impart a high viscosity to the sample. The above changes in viscosity are repeatable, and the sample can be reversibly cycled back and forth between low and high viscosity states. Our photoreversible PR fluids can be easily replicated in any industrial or academic lab, and it is hoped that these “smart” fluids will eventually find a host of applications.
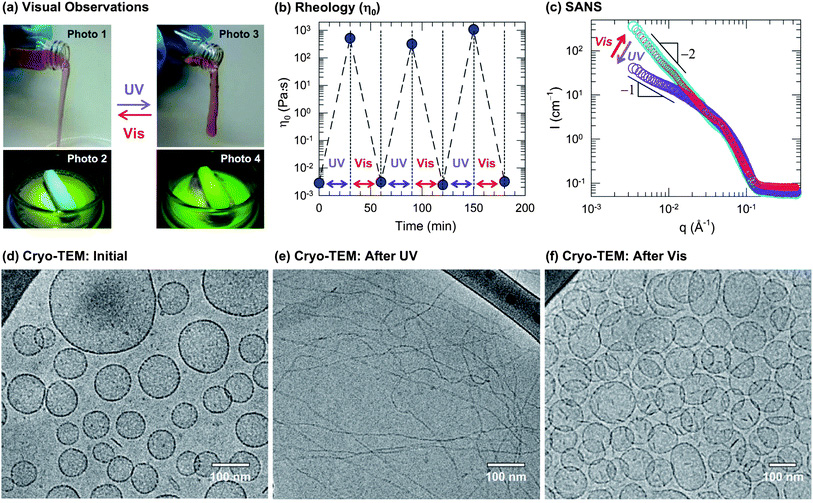
Figure: Reversible photorheological response and its nanostructural origin. A sample containing 40 mM EHAC, 20 mM ACA, and 22 mM NaOH was used for (a), (b) and (d)–(f). (a) Visual observations illustrate the dramatic light-induced changes in fluid properties: initially, the sample is a cloudy, water-like fluid (Photo 1 and 2) whereas UV light transforms it into a transparent viscoelastic and gel-like fluid that shows the tubeless siphon (Photo 3) and rod climbing (Photo 4) effects. This is reversed by irradiation with visible light. (b) The rheology is quantified in terms of the zero-shear viscosityη0. The data show that the sample can be cycled between low and high viscosity states (105-fold difference) by repeated UV and visible light irradiation. (c) SANS spectra on a diluted sample in D2O (10 mM EHAC, 6.5 mM ACA, and 7.2 mMNaOH) reveal a reversible light-induced transition between two types of self-assembled structures: initially, the intensity Ifollows a slope of −2 at low q (cyan circles), which is characteristic of vesicles at low q. Upon UV irradiation, the slope is decreased and approaches −1 (violet circles), which is indicative of cylindrical (wormlike) micelles. Upon subsequent visible-light irradiation, the original spectrum is recovered (red circles). The above structural transition is confirmed bycryo-TEM. A typical image of the initial sample (d) reveals discrete unilamellar vesicles, whereas after UV irradiation (e) the sample is found to contain long, entangled wormlike micelles, and finally, after subsequent visible-light irradiation (f) the sample reverts to the vesicle state.