The influence of the health condition of the cow on the properties of the milk
The Health condition of the cow influences the quality of milk. The quality of the milk is determined by the concentration and the content of the proteins in the milk. Milk from healthy cow contains proteins such as αs2-casein, αs1-casein, β-casein, κ-casien α,-lactoglobulin and β-lactoglobulin. All the samples were determined as a food grade. However, some of the samples were taken from cows, which were infected by bacteria such as E. coli and Streptococcus.
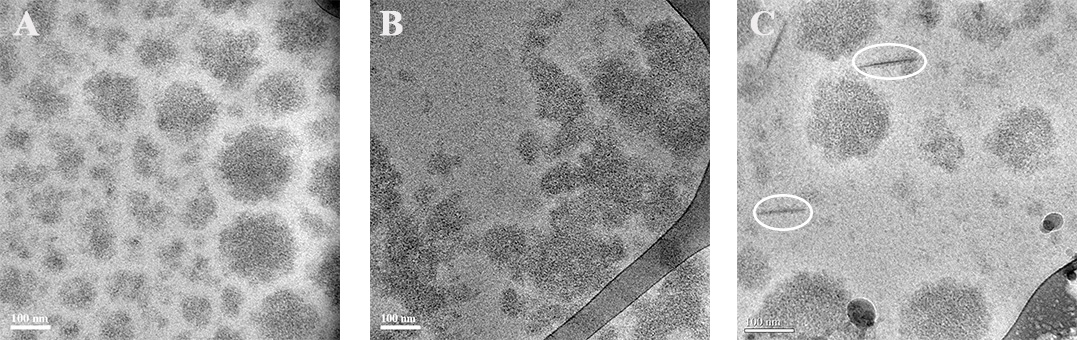
The research focuses on the differences between the content and type of proteins and the size of micelles and fat globules in the samples. This study may show that even though all milk samples were considered as a food grade, the fact that the cows were infected by bacteria has influenced the quality of the milk.
Figure 1. Cryo-TEM images of milk micelles. (A) Milk micelles from healthy cow. (B) Disassembly of milk micelles from a cow with infection of Streptococcus. (C) Milk micelles with needles (in circle) from a cow with infection of Streptococcus
Mixed micellization and fibrillization of casein proteins
Caseins, which comprise the main protein elements (~80%) of mammalian milk, play an important role in cell uptake of physiologically important ions such as calcium and phosphate. These proteins are unfolded, amphiphilic, and self-assemble into core-shell micelles. Kappa-casein (kCN) comprises a predominantly hydrophobic block at the N-terminal end, and a hydrophilic block at the C-terminal end. It also self-organizes into filaments resembling amyloid fibrils. Beta-casein (bCN) has a predominantly hydrophobic block at the N-terminal end, and a hydrophilic block at the C-terminal end. Both of the caseins are characterized by negative net charges at neutral pH.
We examine the associative behavior of both native kCN (N-kCN) and reduced kCN (R-kCN) and their interactions with bCN as a function of temperature and time, with emphasize on the mechanism by which bCN inhibits kCN fibrillization. Cryogenic-transmission electron microscopy (cryo-TEM), Isothermal Titration Calorimetry (ITC), z-potential measurements, and Small-Angle x-Ray Scattering (SAXS) are employed.
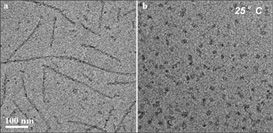
We clearly show that micellization and fibrillization in both pure kCN and in kCN mixtures with βCN are competing processes. While in pure kCN micellization is less favorable than fibrillization in kCN/βCN mixed micelles successfully compete and reduces or eliminates fibril formation (Figure 1).
Figure 1. Cryo-TEM images of (a) N-kCN and (b) mixture of N-kCN and βCN (mole ratio 1:1) at 25°C.
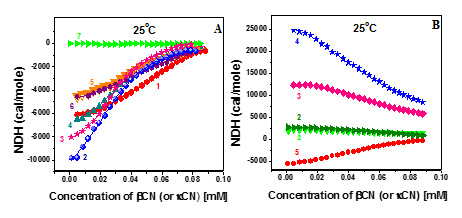
An endothermic character of demicellization of individual N-kCN and its mixtures with βCN, opposite to exothermic demicellization of individual βCN and its mixtures with R-kCN at the same temperatures, were revealed.
Figure 2. Reaction enthalpy of R-kCN, and R-kCN/βCN mixtures (mole ratio 1:1) (A); N-kCN and N-kCN/βCN mixtures (mole ratio 1:1) (B) as a function of incubation time.
Therefore, we show that R-kCN cannot to serve as a full model for N-kCN.