Ongoing Projects
- 2016-2020 Israel Science Foundation (ISF). Lipid self-assembly into unilamellar and multilamellar nanotubes: structure, pathways and kinetics.
- 2016-2020 H2020-NMBP-2016, Health. Smart multifunctional GLA-nanoformulation for Fabry disease. 10 research groups, Coordinator Dr. Nora Ventosa, Spain. 5,844,500€.
- 2019-2021 Israel Dairy Board. Influence of intramammary infection by Streptococci and Escerichia coli on the coagulation properties of milk.
- 2019-2020 Materna, Following structure formation during the digestion of infant formulas by CryoEM
- 2020-2023 The Fifth Russian-Israeli Scientific Research Program, Solid-lipid multifunctional nanocarriers for cancer diagnostic and treatment. Prof. Dganit Danino & Prof. Marina Koroleva.
Structure-function characterization of membrane binding proteins
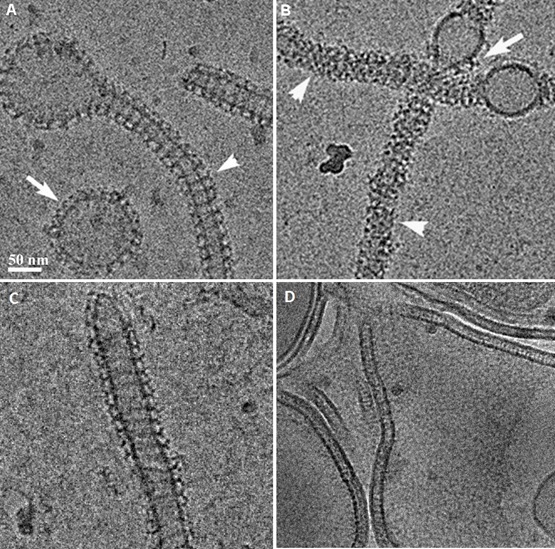
Structure-function relations of three members of the dynamin family : The study defined the GTPase, self-assembly, and membrane binding activities of each protein, aiming to better understand the different mechanisms of action. We found that different proteins of the dynamin family form distinct highly ordered protein-membrane complexes that are customized to fit their diverse biological functions: Dynamin facilitates fission of vesicles (A&B), MxA forms stable membrane-association tubes as storage form in the pre infectious stage (C), Mgm mediates fusion of the inner mitochondrial membrane and maintenance of cristae structures (D).
Membrane remodeling by F-BAR domain proteins
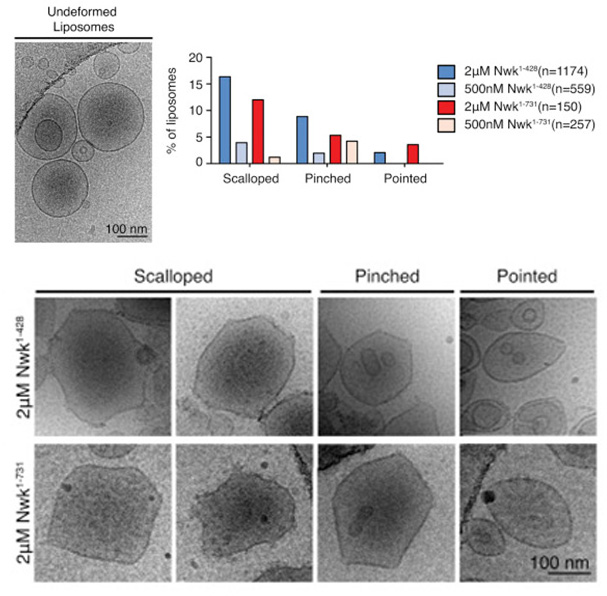
Membrane remodeling is critical for cellular processes such as cargo trafficking, signaling, cell motility, and organelle biogenesis, and it requires the concerted action of scores of proteins that bind and actively shape cellular membranes. F-BAR domain proteins regulate and sense membrane curvature by interacting with negatively charged phospholipids and assembling into higher-order scaffolds. However, regulatory mechanisms controlling these interactions are poorly understood. Here, we show that Drosophila Nervous Wreck (Nwk) is autoregulated by a C-terminal SH3 domain module that interacts directly with its F-BAR domain. Surprisingly, this autoregulation does not mediate a simple “on-off” switch for membrane remodeling. Instead, the isolated Nwk F-BAR domain efficiently assembles into higher-order structures and deforms membranes only within a limited range of negative membrane charge, and autoregulation elevates this range. Thus, autoregulation could either reduce membrane binding or promote higher-order assembly, depending on local cellular membrane composition. Our findings uncover an unexpected mechanism by which lipid composition directs membrane remodeling. The Nwk F-BAR self-assembles into zigzags, distinct from canonical F-BAR proteins, and thus induces membrane scallops and ridges rather than membrane tubules.
Figure: Cryo-EM of control and Nwk deformed liposomes. Both purified Nwk1–428 (F-BAR) and Nwk1–731 (full length) induce membrane scalloping, pointing, and pinching of 10% PI(4,5)P2 liposomes. Scale bar, 100 nm. Bar graph summarizes vesicle morphology after 30-min incubation of Nwk1–428 or Nwk1–731 (2 μM and 500 nM) with 0.3 mM [DOPC:DOPE:DOPS:PI(4,5)P2] = 70:15:5:10 liposomes. n represents the number of liposomes examined.
Mechanistic aspects in the self-assembly of lipids into nano- and microtubes
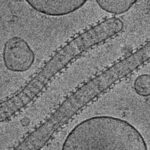
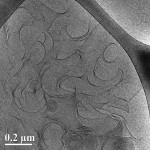
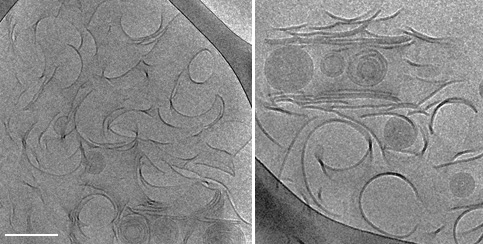
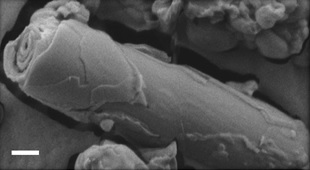
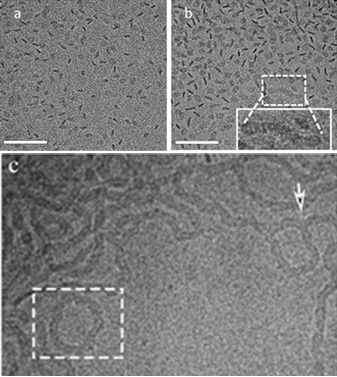
The self-assembly of biological and synthetic based lipids into micro- and nano- structures has been the subject of intense study in past years, both for basic research and for potential applications. Lipids as phosphatidylcholine (PC), phosphatidylethanolamine (PE) and phosphatidylserines (PS) that are common in the plasma membranes, can exhibit a variety of lamellar phases in aqueous solution. Some of them as well as synthetic or mixed-lipid systems can form chiral aggregates in the form of cylindrical tubes, to minimize their free energy requirement. Luba’s research focuses on the self-assembly of 1,2-bis(10,12-tricosadiynoyl)-sn-glycero-3-phosphocholine (DC8,9PC), a synthetic lipid comprising diacetylene moieties in its tail, which presents a distinct aggregation behavior into multilamellar structures. Using advanced electron microscopy techniques as the main research tools, we were able to follow the evolution and maturation of the structures into tubes.
Figure: Intermediate twisted, curled and stacked membrane fragments formed from DC8,9PC in 70% EtOH/ 30% H2O, during a fast cooling process 25°C.
Nanostructure and mechanism of one-dimensional self-assembly of amphiphilic molecules
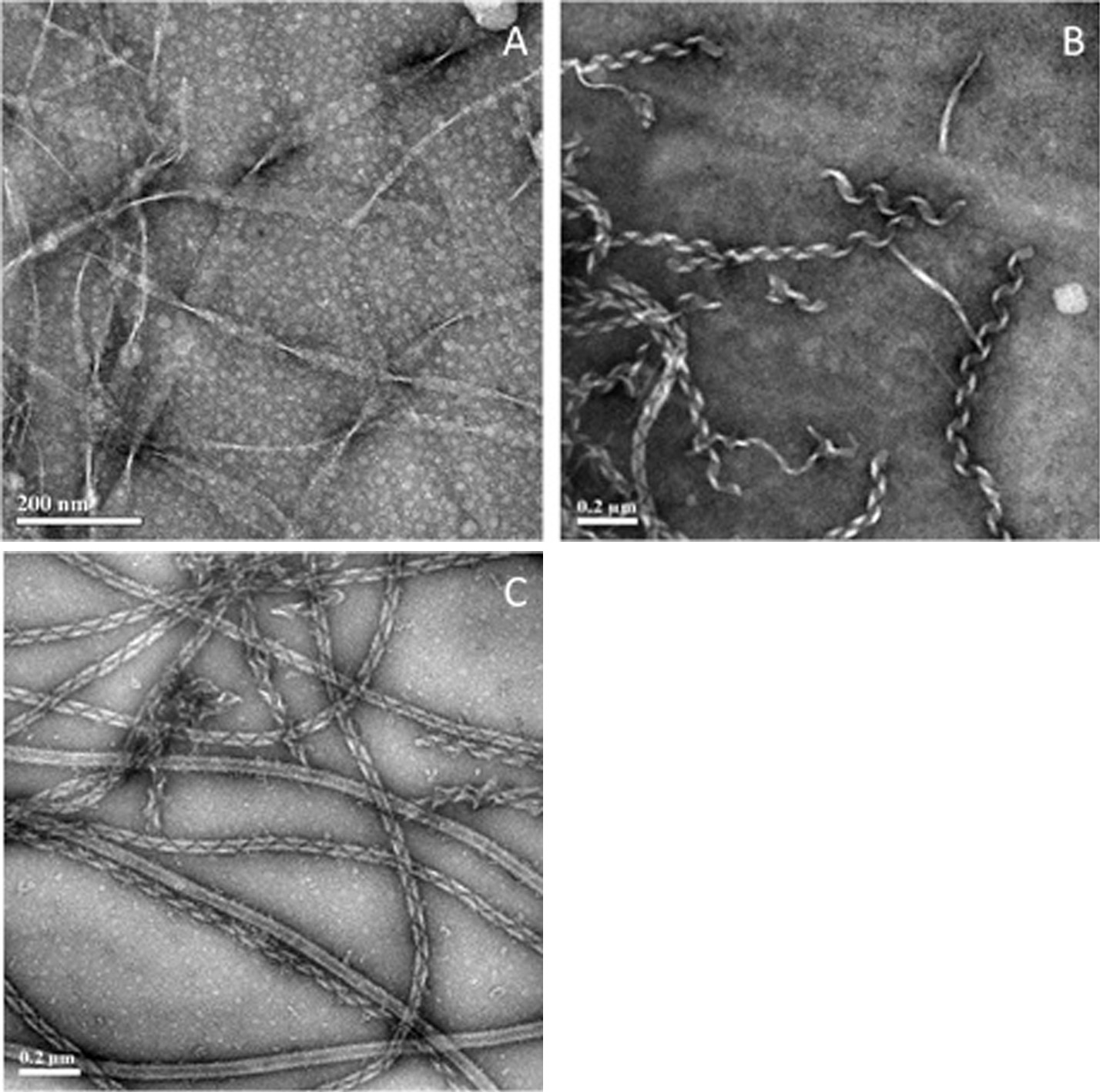
Under the wide scope of self-assembly, there are certain types of structures which are much less understood than others. One such class of structures comprises 1-dimensional (1-D) assemblies, which encompass fibers, ribbons and tubes. 1-D structures can be associated with many human amyloid diseases, including Alzheimer, type II diabetes and multiple sclerosis, and also with processes like collagen self-organization into triple helix fibers and the formation of nanotubes. 1-D assemblies have important advantages, e.g., mechanical rigidity, strength, stability and build-in functionality, which make them useful in various nanotechnology fields. The morphology of 1-D assemblies is influenced by the molecular structure of the building blocks and experimental conditions. Studying different building blocks, such as various amphiphilic molecules, can reveal universal information about 1-D self-assembly, which is not system-dependent.
Figure 1. NS-TEM images of amphiphilic molecules forming 1-D structures. (A) Solution of the amphiphilic peptide decanoyl-lysyl-aminodecanoyl-lysyl at pH 8, incubated at room temperature for a few minutes after NaOH addition. The amphphilic surfactant C2H4-1,2-((CH3)2N+C18H37)2 in water at 35°C after 5 days of incubation (B) and after 1 month of incubation (C).
Nanocarriers based on self-assembled BETA-casein micelles
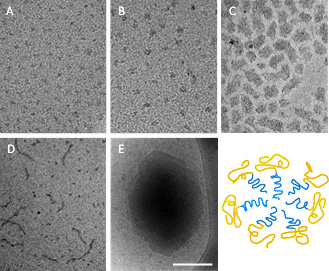
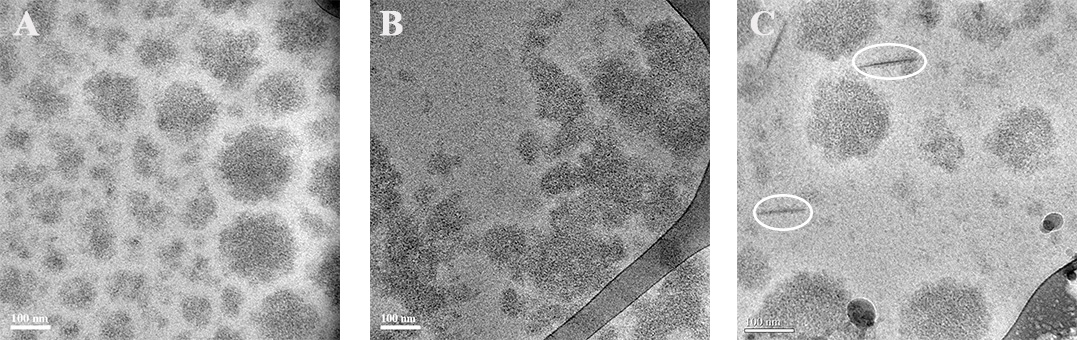
β-Casein is an amphiphilic protein that self-organizes above the critical micellar concentration (CMC) into well-defined core-shell micelles (Fig. 1A). In my research, I develop these protein micelles as efficient nanocapsules of drugs for oral delivery applications. Such core-shell polymeric micelles are considered very attractive candidates for drug delivery, as potentially they can encapsulate large loads of water-insoluble as well as amphiphilic drugs at the micelles’ hydrophobic core (Fig. 1B) or core–shell interface (Fig. 1 C and D), and provide enhanced stability, prolonged circulation times and controlled release.
At the same time, β-casein has all the essential molecular characteristics of an excellent emulsifying agent and polymeric stabilizer, thus it is also utilized for drug stabilization in the form of nanocrystals (Fig. 1E).
Fig. 1: β-Casein micelles: empty (A) and loaded with various poorly water-soluble drugs (B-E), showing its excellent and versatile abilities as a drug delivery platform. Celecoxib is solubilized, stabilized and protected in the micelle core at an amorphous state (B); Loading with ibuprofen leads to aggregation (C) or mixed micellization (D); Budesonide is stabilized as nanocrystals of well-defined size and shape. Bar = 100nm (A-B) and 200 nm (C-E).
Novel Cochleates for Nano-Medicine
Cochleates are lipid microstructures, consisting of negatively charged phospholipid bilayers, in which the planar phospholipid bilayer is induced to wrap around itself to form elongated multilayered cylindrical structures. A novel type of cochleate, able to microencapsulate water-soluble cationic drugs or peptides into its inter-lipid bilayer space, may be formed through interaction between negatively charged lipids and drugs or peptides acting as the inter-bilayer bridges instead of multi-cationic metal ions. The main goal of the research is to explore the conditions required to engineer efficient and stable nano-structures consisting of different species of therapeutic agents with a variety of lipid mixtures mimicking the cytoplasmic membrane of bacteria. Ultimately, to reveal the mechanisms dictating the drug-lipid conjugates assembly and develop advanced methodologies for controlling and characterizing the structures in terms of size, structure and morphology.
The influence of the health condition of the cow on the properties of the milk
The Health condition of the cow influences the quality of milk. The quality of the milk is determined by the concentration and the content of the proteins in the milk. Milk from healthy cow contains proteins such as αs2-casein, αs1-casein, β-casein, κ-casien α,-lactoglobulin and β-lactoglobulin. All the samples were determined as a food grade. However, some of the samples were taken from cows, which were infected by bacteria such as E. coli and Streptococcus.
The research focuses on the differences between the content and type of proteins and the size of micelles and fat globules in the samples. This study may show that even though all milk samples were considered as a food grade, the fact that the cows were infected by bacteria has influenced the quality of the milk.
Figure 1. Cryo-TEM images of milk micelles. (A) Milk micelles from healthy cow. (B) Disassembly of milk micelles from a cow with infection of Streptococcus. (C) Milk micelles with needles (in circle) from a cow with infection of Streptococcus
Mixed micellization and fibrillization of casein proteins
Caseins, which comprise the main protein elements (~80%) of mammalian milk, play an important role in cell uptake of physiologically important ions such as calcium and phosphate. These proteins are unfolded, amphiphilic, and self-assemble into core-shell micelles. Kappa-casein (kCN) comprises a predominantly hydrophobic block at the N-terminal end, and a hydrophilic block at the C-terminal end. It also self-organizes into filaments resembling amyloid fibrils. Beta-casein (bCN) has a predominantly hydrophobic block at the N-terminal end, and a hydrophilic block at the C-terminal end. Both of the caseins are characterized by negative net charges at neutral pH.
We examine the associative behavior of both native kCN (N-kCN) and reduced kCN (R-kCN) and their interactions with bCN as a function of temperature and time, with emphasize on the mechanism by which bCN inhibits kCN fibrillization. Cryogenic-transmission electron microscopy (cryo-TEM), Isothermal Titration Calorimetry (ITC), z-potential measurements, and Small-Angle x-Ray Scattering (SAXS) are employed.
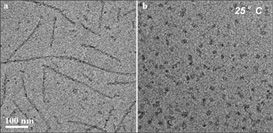
We clearly show that micellization and fibrillization in both pure kCN and in kCN mixtures with βCN are competing processes. While in pure kCN micellization is less favorable than fibrillization in kCN/βCN mixed micelles successfully compete and reduces or eliminates fibril formation (Figure 1).
Figure 1. Cryo-TEM images of (a) N-kCN and (b) mixture of N-kCN and βCN (mole ratio 1:1) at 25°C.
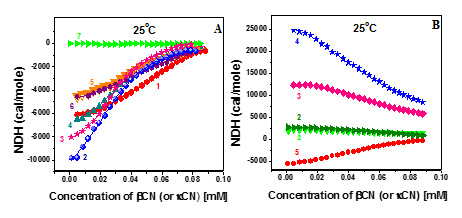
An endothermic character of demicellization of individual N-kCN and its mixtures with βCN, opposite to exothermic demicellization of individual βCN and its mixtures with R-kCN at the same temperatures, were revealed.
Figure 2. Reaction enthalpy of R-kCN, and R-kCN/βCN mixtures (mole ratio 1:1) (A); N-kCN and N-kCN/βCN mixtures (mole ratio 1:1) (B) as a function of incubation time.
Therefore, we show that R-kCN cannot to serve as a full model for N-kCN.
From Discs to Ribbons Networks
At the critical micelle concentration (CMC), amphiphiles self-assemble into spherical micelles, typically followed by a transition at the second CMC to cylindrical micelles that are uniform in width but are polydispersed in length and have swollen ends. We show a new structural path of self-assembly that is based on discoidal (coin-like), rather than spherical, geometry; the nonionic sterol ChEO10 is shown to form monodisperse equilibrium disc assemblies at the first CMC, transitioning at the second CMC into flat ribbons that (like the cylindrical micelles) have uniform width, polydispersed length, and swollen ends. Increase in ChEO10 concentration or the temperature leads to ribbon elongation, branching, and network formation. This self-assembly path reveals that (1) surfactants can form equilibrium nonspherical assemblies at the CMC and (2) aggregate progression around the second CMC is similar for the disc and sphere geometries.
Figure: At 30 °C, 1 wt% ChEO10 disc-like aggregates are formed (a). (b) 2 wt %: coexistence between disc-like assemblies and some elongated, ribbon structures. The inset highlights a short ribbon with swollen endcaps. (c) At 50 °C, 1 wt%, network creation through three-fold junctions (white arrows) between ribbon segments, as well as rings (enclosed in rectangles). Scale bar = 100 nm
Internalization of Silica Nanoparticles into Fluid Liposomes: Formation of Interesting Hybrid Colloids
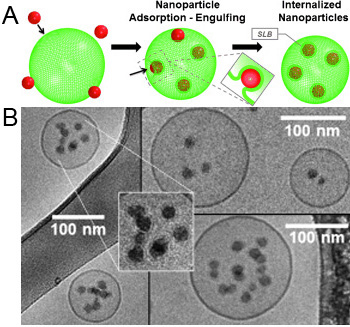
The formation of hybrid materials consisting of membrane-coated silica nanoparticles (SiNPs) concentrated within small unilamellar vesicles (SUVs) of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) is described. They are formed by a simple self-assembly process resulting from invagination of the SiNPs into the SUVs and subsequent vesicle fusion, thereby retaining an almost constant size. This process was followed under conditions where it proceeds slowly and could be analyzed in structural detail. The finally formed well-defined SiNP-filled vesicles are long-time stable hybrid colloids and their structure is conveniently controlled by the initial mixing ratio of SiNPs and vesicles.
Figure: Mechanism of NP internalization. (A) Scheme depiction – hydrophilic NPs adsorb to the lipid are wrapped and finally internalized into the fluid liposomes, thereby forming supported lipid bilayer (SLB) NPs (the inset depicts the process of invagination). (B) Cryo-TEM micrographs showing NP internalized into vesicles. The enlargement shows the presence of SLBs around internalized SiNPs.
A simple route to fluids with photo-switchable viscosities based on a reversible transition between vesicles and wormlike micelles
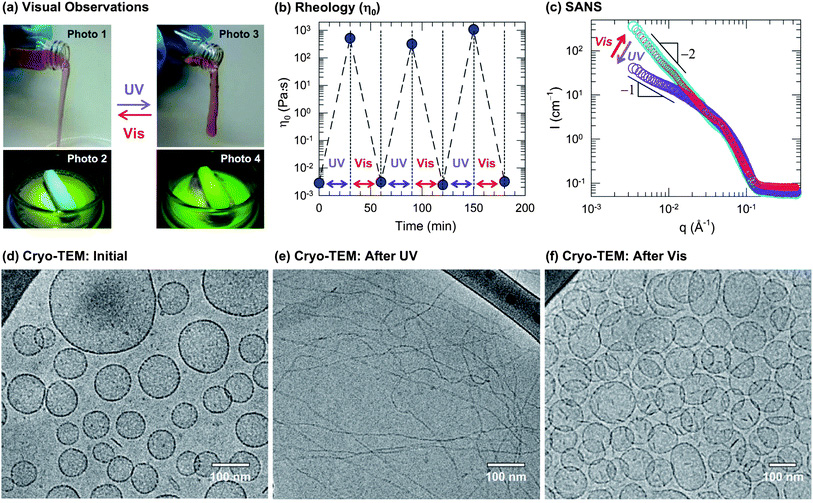
Recently, there has been much interest in photorheological (PR) fluids, i.e., fluids whose rheological properties can be tuned by light. In particular, there is a need for simple, low-cost PR fluids that can be easily created using inexpensive, commercially available ingredients and that show substantial, reversible changes in rheology upon exposure to different wavelengths of light. Towards this end, we report a class of photoreversible PR fluids prepared by combining the azobenzene derivative 4-azobenzene carboxylic acid (ACA) (in its salt form) with the cationic surfactant erucyl bis(2-hydroxyethyl)methyl ammonium chloride (EHAC). We show that certain aqueous mixtures of EHAC and ACA, which are low-viscosity solutions at the outset, undergo nearly a million-fold increase in viscosity when irradiated with UV light. The same solutions revert to their initial viscosity when subsequently exposed to visible light. Using an array of techniques including UV-vis and NMR spectroscopies, small-angle neutron scattering (SANS) and cryo-transmission electron microscopy (cryo-TEM), we have comprehensively characterized these PR fluids at the molecular, nanostructural, and macroscopic scales. Initially, EHAC–ACA are self-assembled into unilamellar vesicles, which are discrete container structures and give the sample a low viscosity. Upon exposure to UV light, ACA undergoes a trans to cis photoisomerization, which alters the geometry of the EHAC–ACA complex. In turn, the molecules self-assemble into a different structure, viz. wormlike micelles, which are long, entangled chains and impart a high viscosity to the sample. The above changes in viscosity are repeatable, and the sample can be reversibly cycled back and forth between low and high viscosity states. Our photoreversible PR fluids can be easily replicated in any industrial or academic lab, and it is hoped that these “smart” fluids will eventually find a host of applications.
Figure: Reversible photorheological response and its nanostructural origin. A sample containing 40 mM EHAC, 20 mM ACA, and 22 mM NaOH was used for (a), (b) and (d)–(f). (a) Visual observations illustrate the dramatic light-induced changes in fluid properties: initially, the sample is a cloudy, water-like fluid (Photo 1 and 2) whereas UV light transforms it into a transparent viscoelastic and gel-like fluid that shows the tubeless siphon (Photo 3) and rod climbing (Photo 4) effects. This is reversed by irradiation with visible light. (b) The rheology is quantified in terms of the zero-shear viscosityη0. The data show that the sample can be cycled between low and high viscosity states (105-fold difference) by repeated UV and visible light irradiation. (c) SANS spectra on a diluted sample in D2O (10 mM EHAC, 6.5 mM ACA, and 7.2 mMNaOH) reveal a reversible light-induced transition between two types of self-assembled structures: initially, the intensity Ifollows a slope of −2 at low q (cyan circles), which is characteristic of vesicles at low q. Upon UV irradiation, the slope is decreased and approaches −1 (violet circles), which is indicative of cylindrical (wormlike) micelles. Upon subsequent visible-light irradiation, the original spectrum is recovered (red circles). The above structural transition is confirmed bycryo-TEM. A typical image of the initial sample (d) reveals discrete unilamellar vesicles, whereas after UV irradiation (e) the sample is found to contain long, entangled wormlike micelles, and finally, after subsequent visible-light irradiation (f) the sample reverts to the vesicle state.